|
by Frank J. Jochem Marine Biology, in press
Cytometric quantification of cellular fluorescence upon cleavage of fluorescein diacetate (FDA) is presented as a sensitive and rapid technique to assess phytoplankton metabolic activity during exposure to prolonged darkness of 10-12 days. Two distinct types of metabolic response upon darkness are distinguished: Type I cells (Brachiomonas submarina, Pavlova lutheri, Chrysochromulina hirta) adapt to prolonged darkness by reducing their metabolism to a lower level of activity (~10% of initial in P. lutheri, C. hirta, ~0.5% in B. submarina) within few days whereas type II cells (Prymnesium parvum, Bacteriastrum sp., unidentified pennate diatom) continue with unchanged activity. Type I cells were able to maintain their initial cell abundance and commenced rapid cell growth upon re-illumination after 12 days of darkness. Among type II cells, diatoms were able to maintain cell abundance and growth capacity as well whereas P. parvum was not. Type I cells are expected to exhibit competitive advantages in environments with frequent or long dark periods. Bacterivory further supports dark survival in C. hirta.
Introduction Although photoautotrophic growth of phytoplankton is light dependent and therefore restricted to the euphotic zone, live algal cells are often encountered well below the lit ocean layers. These cells might have sunken out of the upper water column or occurred in physically displaced surface-near water. Observations of deep phytoplankton assemblages have been presented as evidence for water-mass subduction in the California Current System (Kadko et al. 1991, Wishburn et al. 1991), for example. Murphy & Cowles (1997) reported 50 x 60 km wide patches of live phytoplankton at aphotic depths of 150-200 m off the California shelf that contributed a biomass about 2.5 times the amount of chlorophyll in the overlaying euphotic zone. Especially during winter and spring, phytoplankton of temperate and boreal region can experience quite frequent deep-mixing due to strong winds and thermal convection. The time of withstanding deep-mixing and dark conditions can determine the survival and competitive success of a specific population or species in an environment exhibiting such conditions frequently. Some groups of phytoplankton possess the capability of forming resting cells such as resting spores in diatoms, cysts in dinoflagellates and akinetes in cyanobacteria. The majority of small eukaryotic phytoflagellates, however, do not show such survival stages. Because of their small size of mostly less than 10 µm, they cannot be expected to contain enough energy reserves to survive long periods of darkness without either switching to a heterotrophic mode of nutrition or reducing their metabolic activity to a necessary minimum. The study of physiological response in these species is vital to understand the ecological role of deep-water chlorophyll accumulations. The measurement of fluorescein diacetate (FDA) hydrolysis has been applied to estimate microbial biomass on corniferous needles (Swisher & Carroll 1980), to determine total microbial activity in soil (Schnürer & Roswall 1982), water (Holzapfel-Pschorn et al. 1987) and deep-sea sediments (Köster et al. 1991, Gumprecht et al. 1995), and to differentiate between live and dead/unhealthy cells in mammalian cell cultures (Ross et al. 1989) and microalgae (Gilbert et al. 1992). In connection with pollution studies and using fluorescence microscopy, Bentley-Mowat (1982) first reported that the intensity of fluorescence derived from the cleavage of FDA appeared to depend on the "metabolic vigour" of the cells. Dorsey et al. (1989) optimized the FDA technique for application in flow cytometry; their protocol was adopted here except for minor changes described below. FDA is a nonpolar, nonfluorescent substance which enters the cells freely. Inside the cell, nonspecific esterases, among them lipase and acylase but not acetylcholinesterase (Guilbaut & Kramer 1966), break the FDA molecule into one brightly fluorescing fluorescein and two acetates. Being highly polar, the fluorescein is trapped within cells exhibiting cell membrane integrity and the amount of fluorescence will therefore increase over time depending on the metabolic activity of those esterases. Material and Methods The chlorophyte Brachiomonas submarina, the prymnesiophytes Prymnesium parvum, Pavlova lutheri, and Chrysochromulina hirta (Baltic Sea isolate), the diatom Bacteriastrum sp. and an unidentified pennate Nitzischa-like diatom isolated from the North Atlantic (47°N 20°W, R/V Meteor, Mai 1992) were maintained as batch cultures in f/20 medium in artificial seawater (except for C. hirta in Baltic Sea water) at 18°C and 80 µE m-2 s-1 provided by white fluorescent tubes under a light/dark cycle of 14:10 hrs. For the dark survival experiments, subsamples of late log-phase cultures were inoculated into fresh f/20 medium, placed into darkness at 10°C, and sampled daily at the time of noon under the previous L/D cycle. To test for heterotrophic potentials during dark survival, in experiment I comprising B. submarina, P. lutheri, C. hirta, P. parvum, and the pennate diatom, cultured bacteria were added to a final concentration of 7.5 x 105 ml-1 in parallel incubations to those without bacteria except for the diatom where bacterivory was not expected. In experiment II comprising B. submarina, P. lutheri, and Bacteriastrum sp., parallel incubations with added organics (final conc. 5 µM of glucose and leucin) should test for osmotrophic potentials. A stock solution of FDA (Sigma Chemicals F-7378) of 5 mg ml-1 was made in dimethylsulfoxide (DMSO) and stored at 4°C. The stock solution was thawed (DMSO freezes at 18°C) and diluted 100-fold in distilled water; since FDA is only slightly soluble in aqueous solutions and tends to flocculate at >1 µg ml-1, the stock solution was injected fast into the ice-cold water and mixed quickly. Although the working solution might appear slightly opaque, flocculation was prevented. The working solution was kept on ice to minimize FDA degradation for max. 3 hrs and prepared fresh daily. 100 µl FDA working solution were added to 3 ml of sample in Becton-Dickinson (BD) cytometer tubes kept at room temperature in the dark until measurement. Cells were analyzed by a BD FACSort flow cytometer equipped with a 488 nm argon laser. Phytoplankton cells were identified and distinguished from other particles by gating on 2-parameter-plots of Forward Angle Light Scatter (FSC) versus chlorophyll fluorescence gathered through a 650 nm longpass filter. Green fluorescein fluorescence was measured through a 535±15 nm bandpass filter on a 4-decades log scale. BD Calibrite standard beads with green and red fluorescence, respectively, were used to calibrate the machine settings for identical fluorescence yields of the daily analyses and all FDA fluorescence measurements were taken for all species and all days at the same settings so that FDA fluorescence readings normalized per cell are comparable among the studied species. Results For optimization and standardization of the FDA staining protocol, accumulation of fluorescein was followed continuously for 15 mins after FDA addition in pre-experiments (Fig. 1). FDA readily penetrated into the cells right after addition and green fluorescence increased until 4-5 mins. Thereafter, variation in cellular fluorescence increased and eventually leakage of fluorescein out of the cells further increased the variation and reduced the mean cellular fluorescence. For the subsequent dark survival studies it was decided to take fluorescence readings exactly 5 mins after FDA addition. This timing gave a good compromise of reasonably low variation in fluorescence readings and high fluorescence yields from FDA cleavage. Instrument settings were adjusted so that non-marked cells exhibited a "fluorescence" below 2 relative units on the 4-log scale. Hence, cells with fluorescence readings above this value were considered FDA positive. 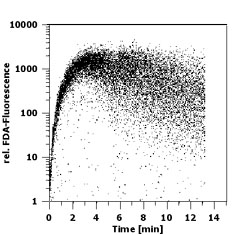 Fig.1: Time course of fluorescence (relative untis)
accumulation upon FDA addition in Brachiomonas submarina as revealed by flow
cytometry
Fig.1: Time course of fluorescence (relative untis)
accumulation upon FDA addition in Brachiomonas submarina as revealed by flow
cytometry
Generally, all cultures exhibited a fairly low variation in their FDA fluorescence at the start of dark incubations (Fig. 2 a,b; first measurements). As the experiments progressed, FDA fluorescence not only decreased but in some cases the coefficient of variation of the fluorescence frequency distributions increased as well as can be seen by broader frequency distributions in fig. 2a. For the presentation of cellular metabolic activity as assessed by FDA fluorescence, both mean fluorescence per cell and the percent of FDA positive cells will be considered. Despite somewhat broader frequency distributions in FDA fluorescence along some of the tested cultures, mean and median values generally displayed the same results.
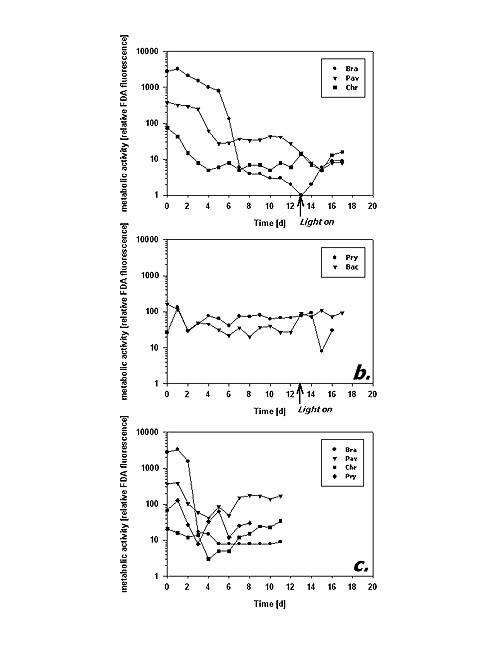
Following the mean cellular fluorescence during dark cultivation reveals two different principles of metabolic response. B. submarina, P. lutheri and C. hirta represent the first type (Fig. 3a): After some reduction in FDA fluorescence during the first days there was a distinct, almost one magnitude downward step after 4 (P. lutheri) to 6 (B. submarina) days in the dark. Subsequently, metabolic activity remained on a more or less constant low level. C. hirta reduced its metabolic activity already during the first 4 days to reach a constant low level thereafter. In contrast, P. parvum and Bacteriastrum sp. kept their metabolic activity at an unchanged magnitude throughout the experiment (Fig. 3b).
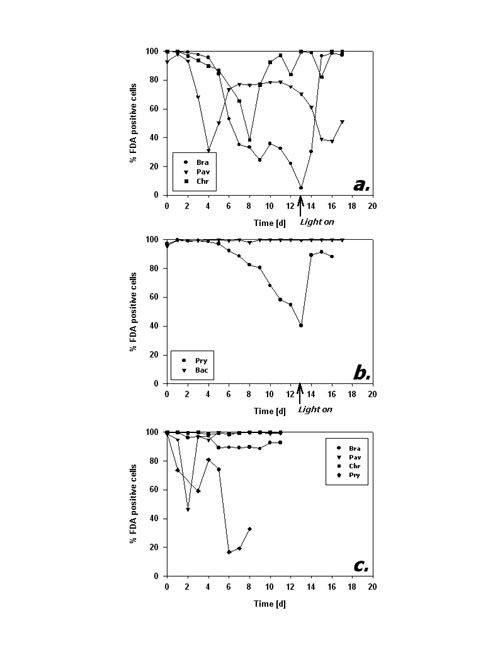
Fig.3: Mean cellular FDA derived fluorescence (relative units) of phytoplankton kept in darkness; a,b – incubation without bacteria, c – incubation with added bacteria; Bra = Brachiomonas submarina, Pav = Pavlova lutheri, Chr = Chrysochromulina hirta, Pry = Prymnesium parvum, Dia = pennate diatom; arrows in a,b indicate time of re-illumination in incubations without bacteria With the addition of bacteria, metabolic activity of C. hirta and P. lutheri initially decreased as described above but increased again after day 4 (Fig. 3c). The comparison of distinct FDA fluorescence frequency distributions in C. hirta (Fig. 2) reveal that without bacteria addition, the mean fluorescence decreased and variation of fluorescence increased, meaning that metabolic activity decreased faster in some cells than in others (Fig. 2a). In the presence of bacteria, the culture kept a narrow frequency distribution throughout the experiment, suggesting that all cells responded equally to the presence of bacteria (fig. 2b). In the other species, the addition of bacteria did alter neither the type of response nor the magnitude of low-level esterase activity in any of the studied species (Fig. 3c). The cultures without added bacteria were re-illuminated after 13 days in the dark. In type I response species, metabolic activity increased upon re-illumination, a response which was most pronounced in B. submarina (Fig. 3a). No change in metabolic activity upon re-illumination was recorded in Bacteriastrum sp. whereas P. parvum exhibited a decrease in FDA fluorescence. The response in the fraction of FDA positive cells was more heterogeneous (Fig. 4a,b): P. lutheri and C. hirta exhibited an initial decrease but a later recovery which reached >90% in the latter and still near 80% in P. lutheri. Both B. submarina and P. parvum experienced a steady decline in the contribution of metabolically active cells throughout darkness which developed faster in the former species; re-illumination caused a fast increase of the FDA+ fraction to >90% within 2 days in both species. Bacteriastrum sp. kept its FDA+ fraction at nearly 100% throughout the experiment. With the addition of bacteria, the FDA+ fraction remained at 90-100% except in P. parvum which showed the same decrease as mentioned above (Fig. 4c).
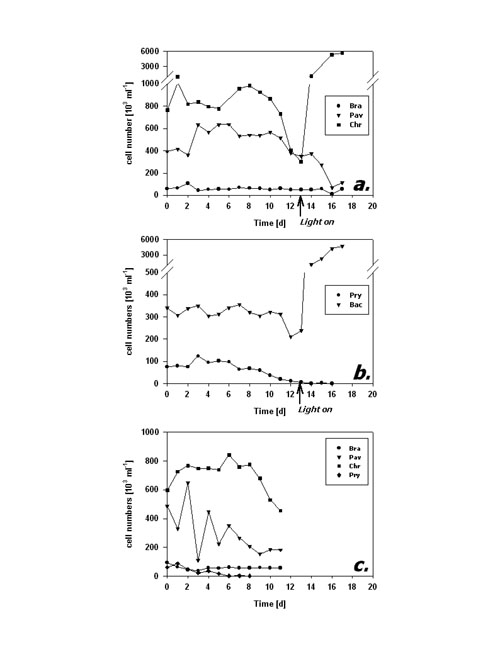
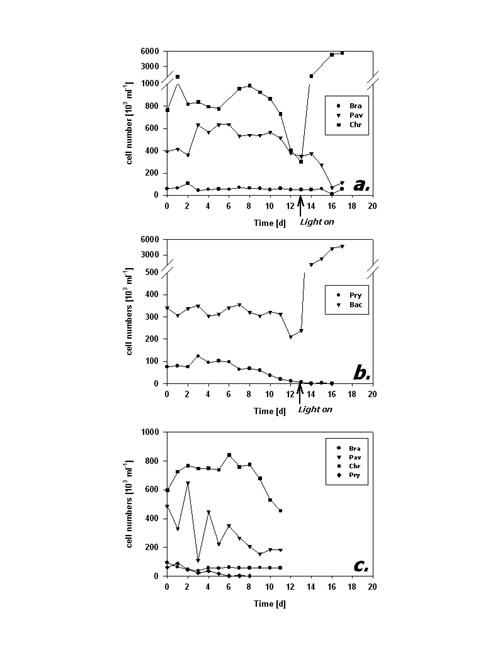
Fig.4: Fraction of FDA positive cells (%) of phytoplankton kept in darkness; a,b – incubation without bacteria, c – incubation with added bacteria; species names as listed in fig 3; arrows in a,b indicate time of re-illumination in incubations without bacteria Cell numbers in type I response species (Fig. 5a) remained quite constant during the first 10 days of darkness. Subsequently, P. lutheri showed a slight and C. hirta a strong decrease in abundance whereas B. submarina abundance still remained constant. Population growth commenced right upon re-illumination in C. hirta but seems to have lagged until day 17, the end of the experiment, in both B. submarina and P. lutheri. Bacteriastrum sp. abundance remained unchanged during darkness and took off right after re-illumination, whereas P. parvum cell numbers declined steadily and did not recover after re-illumination (Fig. 5b). Addition of bacteria did not alter the pattern of cell abundance (Fig. 5c).
Fig.5: Cell abundance (103 ml-1) of phytoplankton kept in darkness; a,b – incubation without bacteria, c – incubation with added bacteria; species names as listed in fig 3; arrows in a,b indicate time of re-illumination in incubations without bacteria The second dark survival experiment generally revealed the same response patterns and time frames (Fig. 6a-c). Metabolic activity decreased after day 3 in P. lutheri and day 7 in B. submarina whereas that of Bacteriastrum sp. remained unchanged. The fraction of FDA+ cells dropped after 3 days in P. lutheri and 7 days in B. submarina but remained near 100% in Bacteriastrum sp. In this experiment, cell numbers of B. submarina decreased slightly, Bacteriastrum sp. abundance remained unchanged, and P. lutheri exhibited a slight growth during the first 4 days in darkness. The addition of glucose and leucin had no influence on neither species’ responses.
Fig.6: a - Mean cellular FDA derived fluorescence (relative units), - b - Fraction of FDA positive cells (%), and – c - Cell abundance (103 ml-1) of phytoplankton kept in darkness; Bra = Brachiomonas submarina, Pav = Pavlova lutheri, Bac = Bacteriastrum sp.; filled symbols represent incubations without, open symbols those with addition of glucose and leucin (final conc. 5 µM) Discussion The FDA method appears as a sensitive, simple and rapid technique to assess cell-to-cell as well as temporal variability in metabolic activity of microalgae. The presented protocol worked well with all tested species of different algal classes and gave reproducible results. Successful application of same or similar protocols has been reported for a total of 44 different phytoplankton species from all major unicellular algal groups (Selvin et al. 1988, Dorsey et al. 1989, Gilbert et al. 1992, Geary et al. 1998, this study) as well as successful bulk measurements of marine field samples to test for the toxicology of weed-killers, insecticides and metals (Gilbert et al. 1992). Since the esterases enabling the FDA assay turn over on a time frame of several hours (Yentsch et al. 1988), this technique seems appropriate to detect changes in metabolic activity on a day-to-day time scale. This is supported by results of Geary et al. (1998) who were able to distinguish cyanobacteria Microcystis aeruginosa grown under different light intensities or under phosphate replete/deplete conditions after 2 days of adaptation; the light-covarying FDA fluorescence corresponded to respectively different cell growth rates. Dorsey et al. (1989) report co-variation of FDA assays and 14CO2-fixation comparing 8 algal species. The FDA assay is, thus, not only helpful to discriminate "healthy" and "stressed" cells but also to quantify subtle responses upon environmental impacts. The advantage of cytometric measurements over fluorescence microscopy or bulk estimates by fluorometry lies in the assessment of minor changes in metabolic activity by the detection of changes in the amount of fluorescence accumulated per time unit on a single cell basis. This ability might even allow to distinguish different metabolic responses upon environmental factors by different species/populations in one given sample. Bulk measurements (e.g. fluorometry, automated measurement in microtitration plates) must account for fluorescence originating from spontaneous degradation and/or bacterial cleavage of FDA as well. These effects were not important to cytometric analyses as only fluorescein fluorescence related to individual algal cells was recorded. This specific advantage of flow cytometry lets changes in FDA cleavage upon addition of bacteria during dark survival be discussed as phagotrophic potential of the studied algae. The analysis of metabolic activity revealed two distinct types of physiological response upon prolonged darkness among the studied phytoflagellates. The species assigned to type I (B. submarina, P. lutheri, C. hirta) seem to recognize the problem of darkness and energy limitation and react by reducing their metabolic activity within few days. Thereby, these species were able to sustain their population abundance throughout a fortnight in the dark. It shall be noted that the result of lower cellular FDA fluorescence only applies to cells acknowledged as FDA positive. Therefore, the type I response represents the metabolic response in active cells only and is not influenced by the fact that the fraction of active (FDA+) cells decreased concomitantly. The second type of response (P. parvum, Bacteriastrum sp., pennate diatom) lacks an adjustment in metabolic activity upon darkness. In the case of P. parvum, going on "as usual" results in constant FDA readings but an inevitable decrease in cell abundance. Although the fraction of FDA positive cells increases drastically upon re-illumination after a fortnight of darkness, it seems that the surviving cells need the new energy to refill their exhausted cellular reserves before they can eventually divide. Therefore, in contrast to type I species, re-illumination did not result in population growth. The two diatoms exhibited a peculiar pattern in that their metabolic activity did not change and almost all cells remained metabolically active. Still they were able to sustain their cell abundance in the dark and to commence rapid population growth upon re-illumination. Diatoms, particularly polar species that are exposed to long winter darkness, are known for their dark survival potentials (Antia & Cheng 1970, Palmisano & Sullivan 1982). Their strategies comprise reduction of metabolism (French & Hargraves 1980), formation of resting spores (Durbin 1978, Doucette & Fryxell 1983) or resting cells without morphological differences to vegetative cells (Anderson 1975, Hargraves & French 1983), and facultative heterotrophy (Hellebust & Lewin 1977). Neither of the studied species displayed signs of resting spore formation, which besides morphological changes would have resulted in severely reduced FDA fluorescence as well. 8 diatoms from the Southern Ocean (Peters & Thomas 1996) and 3 diatoms from the North Sea (Peters 1996) survived up to 10 months of darkness without spore formation and kept their potential for high photosynthesis during dark incubation. Thalassiosira weissfloggii survived 2 months of darkness without spore formation and commenced exponential growth upon re-illumination (Murphy & Cowles 1997); the authors suggest that both the photochemical apparatus and biochemical carbon fixation pathways remained functional and >80% of cells remained metabolically active as microscopically derived from FDA assays. From POC measurements they further assume that T. weissfloggii utilized organic carbon during dark survival. In the present experiment, the inability to take up added organics does not suggest heterotrophy. This observation does not, however, exclude a potential for heterotrophy. The conducted dark experiment might have been too short to yield a physiological state of deprivation to initiate heterotrophy, and energy reserves might have been sufficient to sustain unchanged metabolic activity without biasing the ability to commence rapid cell division upon re-illumination. The nature of the physiological mechanism providing remarkable dark survival capabilities and sustaining unreduced metabolic activity at the same time remains still to be resolved. From their results depicted, the prasinophyte strain CCMP W 48-23 can also be assigned to type I (Dorsey et al. 1989). Among three dinoflagellates from the Ria de Vigo, Spain, tested for dark survival Protogonyaulax affinis was unable to survive for a few days, Gymnodinium catenatum took advantage of cyst formation but Prorocentrum lima was still alive after 3 weeks (Selvin et al. 1988); its lower FDA fluorescence in the dark than in the light as drawn from qualitative microscopic analyses makes this species likely to be of type I as well and the most successful dinoflagellate in their dark survival assay. It can be assumed that type I species will gain a higher competitive advantage over type II species in environments where long and/or frequent dark conditions might be encountered. Reduction in metabolic activity to preserve scarce energy resources is essential for survival here. Type II algae would eventually run out of energy and grow themselves to death. Since high metabolic activity can be re-gained pretty fast at least in some type I species (see fig. 3a, notably B. submarina; also W 48-23 in Dorsey et al. 1989) a type II strategy seems also not advantageous in environments of short dark periods (such as frequent shallow mixing that will return cells to the surface soon). In addition to energy saving, energy scavenging can be a complementary survival strategy. Previous studies showed that phytoplankton at the lower boundary of the euphotic zone might supplement a part of their carbon requirement by osmotrophy (Vincent & Goldman 1980) or phagotrophy (Bird & Kalff 1989). The more "phytoflagellates" were studied, the more turned out facultative or obligate heterotrophic. Often, heterotrophic modes of nutrition in phytoplankton is tuned by darkness, as for example shown for the dinoflagellate Heterocapsa triquetra (Legrand et al. 1998). Among the species studied here, C. hirta exhibited clear signs of enhanced dark survival and higher metabolic activity when bacteria were added to the dark culture, pointing towards bacterivory in this species (see fig. 2b). Several species of Chrysochromulina, for example C. brevifilum (Jones et al. 1995) and C. polylepis (Nygaard & Tobiesen 1993, Rhodes et al. 1994), exhibited bacterivory under light and/or nutrient limitation whereas phagotrophy was absent in C. quadrikonta and C. camella (Rhodes et al. 1994). Phagotrophy in C. hirta was previously documented by Kawachi et al. (1991). Providing that the FDA results in fact reveal bacterivory in C. hirta, this species thus exhibits a double competitive advantage for dark survival: type I metabolic activity response and the ability to utilize other than light-dependent resources. Whereas phagotrophy was demonstrated in Prymnesium patelliferum (Tillmann 1998) and P. parvum (Nygaard & Tobiesen 1993), the here studied culture of P. parvum showed no sign of darkness-induced phagotrophy. Also B. submarina seemed not to utilize bacteria or organics though such growth capabilities have been reported (Tsavalos & Day 1994). Whereas live phytoplankton accumulations well below the euphotic zone seem to occur quite frequently in different parts of the world’s oceans, we still lack a profound knowledge of their origin, species composition and the physiological adaptations of the involved algae. The extended deep-water patch of chlorophyll off the California shelf (Murphy & Cowles 1997) was not taxonomically analyzed but the authors assume that it originated from a sedimented diatom-bloom. High chlorophyll accumulation at 150 m depth well below the euphotic zone in the Polar Front of the Southern Ocean in austral spring 1992 could be assigned to diatoms as well, namely Corethron cryophilum (Jochem & Meyerdierks unpubl.). Algal communities living and proliferating at aphotic and anoxic depths in the central Baltic Sea, however, were composed of various <10 µm phytoflagellates, some resembling Chrysochromulina sp. (Detmer et al. 1993). The cytometric FDA protocol might provide a useful tool to further investigate the physiology of algal cells in aphotic depths and may reveal the ubiquity of the two established types of metabolic adaptation to dark survival. This task is further eased by the recent development of a protocol for cryopreservation of FDA-labeled phytoplankton cells (Faber et al. 1997). Acknowledgments This work was partly funded by German Research Council DFG Jo 192/5-1. Literature Anderson OR (1975) The ultrastructure and cytochemistry of resting cell formation in Amphora coffaeformis (Bacillariophyceae). J Phycol 11: 272-281 Antia NJ, Cheng JY (1970) The survival of axenic cultures of marine planktonic algae from prolonged exposure to darkness at 20°C. Phycologia 9: 179-183 Bentley-Mowat JA (1982) Application of fluorescence microscopy to pollution studies on marine phytoplankton. Bot Mar 25: 203-204 Bird DF, Kalff J (1989) Phagotrophic sustenance of a metalimnetic phytoplankton peak. Limnol Oceanogr 34: 155-162 Detmer AE, Trenkel V, Giesenhagen HC, Auf dem Venne H, Jochem, FJ (1993) Phototrophic and heterotrophic pico- and nanoplankton in anoxic waters of the Central Baltic Sea. Mar Ecol Prog Ser 99: 197-203 Dorsey J, Yentsch CM, Mayo S, McKenna C (1989) Rapid analytical technique for the assessment of cell metabolic activity in marine microalgae. Cytometry 10: 622-628 Doucette GJ, Fryxell GA (1983) Thalassiosira antarctica: Vegetative and resting stage chemical composition of an ice-related marine diatom. Mar Biol 78: 1-6 Durbin EG (1978) Aspects of the biology of resting spores of Thalassiosira nordenskioeldii and Detonula confervacea. Mar Biol 45: 31-37 French FW, Hargraves PE (1980) Physiological characteristics of plankton diatom resting spores. Mar Biol Lett 1: 185-195 Faber M, Smith LMJ, Boermans HJ, Stephenson GR, Thompson DG, and Solomon KR (1997) Cryopreservation of fluorescent marker-labeled algae (Selenastrum capricornutum) for toxicity testing using flow cytometry. Environ Toxicol Chem 16: 1059-1067 Geary S, Ganf G, Brookes J (1998) The use of FDA and flow cytometry to measure the metabolic activity of the cyanobacteria, Microcystis aeruginosa. Verh Internat Verein Limnol 26: 2367-2369 Gilbert F, Galgani F, Cadiou Y (1992) Rapid assessment of metabolic activity in marine microalgae: Application in ecotoxicological tests and evaluation of water quality. Mar Biol 112: 199-205 Guilbaut GG, Kramer DN (1966) Lipolysis of fluorescein and eosin esters – kinetics of hydrolysis. Analyt Biochem 14: 28-40 Gumprecht R, Gerlach H, Nehrkorn A (1995) FDA hydrolysis and resazurin reduction as a measure of microbial activity in sediments from the south-east Atlantic. Helgoländer Meeresunters 49: 189-199 Hargraves PE, French FW (1983) Diatom resting spores: Significance and strategies. In: Fryxell GA (ed) Survival strategies of the algae. Cambridge Univ, pp 49-68 Hellebust JA, Lewin J (1977) Heterotrophic nutrition. In: Werner D (ed) The biology of diatoms. Blackwell Scientific, pp 169-197 Holzapfel-Pschorn A, Obst U, Haberer K (1987) Sensitive methods for the determination of microbial activities in water samples using fluorigenic substrates. Fresenius Z Analyt Chem 327: 521-523 Jones HLJ, Durjun P, Leadbeater BSC, Green JC (1995) The relationship between photoacclimation and phagotrophy with respect to chlorophyll a, carbon and nitrogen content, and cell size of Chrysochromulina brevifilum (Prymnesiophyceae). Phycologia 34: 128-134 Kadko DC, Washburn LW, Jones BH (1991) Evidence of subduction within cold filaments of the northern California coastal transition zone. J Geophys Res 96: 14909-14926 Kawachi M, Inouye I, Maeda O, Chihara M (1991) The haptonema as a food-capturing device: Observations on Chrysochromulina hirta (Prymnesiophyceae). Phycologia 30: 563-573 Köster M, Jensen P, Meyer-Reil, LA (1991) Hydrolytic activities of organisms and biogenic structures in deep-sea sediments. In: Chrost RJ (ed) Microbial enzymes in aquatic environments. Springer, New York, pp 298-310 Legrand C, Granéli E, Carlson P (1998) Induced phagotrophy in the photosynthetic dinoflagellate Heterocapsa triquetra. Aquat Microb Ecol 15: 65-75 Murphy AM, Cowles TJ (1997) Effects of darkness on multi-excitation in vivo fluorescence and survival in a marine diatom. Limnol Oceanogr 42: 1444-1453 Nygaard K, Tobiesen A (1993) Bacterivory in algae: A survival strategy during nutrient limitation. Limnol Oceanogr 38: 273-279 Palmisano AC, Sullivan CW (1982) Physiology of sea ice diatoms. I. Response of three polar diatoms to a simulated summer-winter transition. J Phycol 18: 489-498 Peters E (1996) Prolonged darkness and diatom mortality. II: Marine temperate species. J Exp Mar Biol Ecol 207: 43-58 Peters E, Thomas DN (1996) Prolonged darkness and diatom mortality. I: Marine Antarctic species. J Exp Mar Biol Ecol 207: 25-41 Rhodes LL, O’Kelly CJ, Hall JA (1994) Comparison of growth characteristics of New Zealand isolates of the prymnesiophytes Chrysochromulina quadrikonta and C. camella with those of the ichthyotoxic species C. polylepis. J Plankton Res 16: 69-82 Ross DD, Joneckis CC, Ordóñez JV, Sisk AM, Wu RK, Hamburger AW, Nora RE (1989) Estimation of cell survival by flow cytometric quantification of fluorescein diacetate / propidium iodide viable cell number. Cancer Res 49: 3776-3782 Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43: 1256-1261 Selvin R, Reguera B, Bravo I, Yentsch CM (1988) Use of fluorescein diacetate (FDA) as a single-cell probe of metabolic activity in dinoflagellate cultures. Biol Oceanogr 6: 505-511 Swisher R, Carroll GC (1980) Fluorescein diacetate hydrolysis as an estimator of microbial biomass on corniferous needle surfaces. Microb Ecol 6: 217-226 Tillmann U (1998) Phagotrophy by a plastidic haptophyte, Prymnesium patelliferum. Aquat Microb Ecol 14: 155-160 Tsavalos AJ, Day JG (1994) Development of media for the mixotrophic/heterotrophic culture of Brachiomonas submarina. J Appl Phycol 6: 431-433 Vincent WF, Goldman CR (1980) Evidence for algal heterotrophy in Lake Tahoe, California-Nevada. Limnol Oceanogr 25: 89-99 Washburn LW, Kadko, DC, Jones BH, Hayward T, Kosro PM, Stanton TP, Ramp S, Cowles TJ (1991) Water mass subduction and the transport of phytoplankton in a coastal upwelling system. J Geophys Res 96: 14927-14945 Yentsch CM, Cucci TL, Mague FC (1988) Profiting from the visible spectrum. Biol Oceanogr 6: 477-492
|
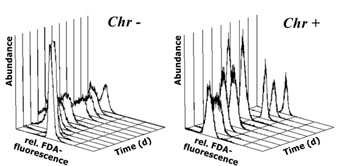 Fig.2: Daily frequency distributions of
FDA derived fluorescence (relative units) in Chrysochromulina hirta
kept in darkness with (Chr+) and without (Chr-) addition of bacteria
Fig.2: Daily frequency distributions of
FDA derived fluorescence (relative units) in Chrysochromulina hirta
kept in darkness with (Chr+) and without (Chr-) addition of bacteria
 Get your free
Adobe Acrobat Reader to download PDF formatted files
Get your free
Adobe Acrobat Reader to download PDF formatted files